Which Best Describes How Heat Energy Moves Within A System
Which best describes how heat energy moves within a system. Energy flows from the greatest concentration of energy to lower concentrations. This is a transformation between mechanical energy and thermal energy. Heat is what happens when thermal energy stops moving between substances.
Which best describes how energy moves within a system. In the home as the air is heated the particles gain heat energy allowing them to move faster warming the cooler particles. Heat is the transfer of thermal energy from a system to its surroundings or from one object to another as a result of a difference in temperature.
Sometimes more than one may occur at the same time. Heat Energy -- Energy exhibited by moving atoms the more heat energy an object has the higher its temperature. Energy decreases as it moves up trophic levels because energy is lost as metabolic heat when the organisms from one trophic level are consumed by organisms from the next level.
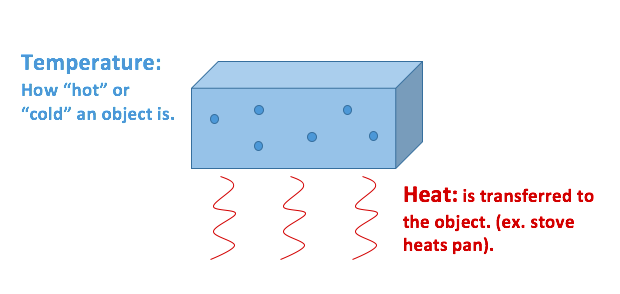
In fact all hot things radiate heat to cooler things. Heat describes the transfer of thermal energy between molecules within a system and is measured in Joules. This is because heat is a transfer of energy.
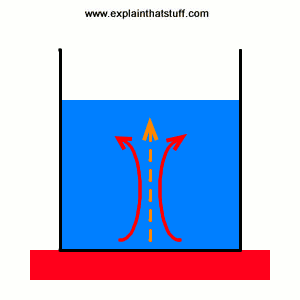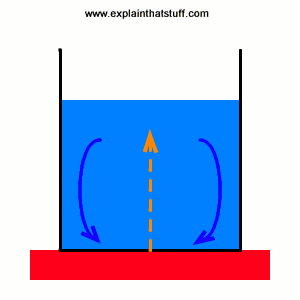
The flow of heat will continue until the two objects are at the same temperature. The kinetic energy in the system is greatest at X. Radiation happens when heat moves as energy waves called infrared waves directly from its source to something else.
The ice and the water within the first minute. Which statement best describes the energy in this system. Nuclear reactions in a reactor produce a lot of thermal energy.
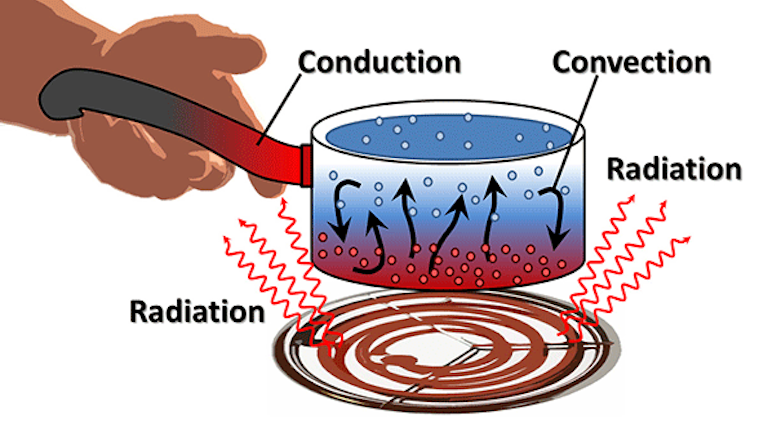
CEnergy flows from energy sinks to energy sources. A pot of water is heated by a stove as the heat energy moves from the stove top to the metal of the pot.
DEnergy flows from energy sources to energy sinks.
The processes are known as conduction convection and radiation. All matter is made from atoms either single ones or. Heat is the transfer of thermal energy from a system to its surroundings or from one object to another as a result of a difference in temperature. Energy decreases as it moves up trophic levels because energy is lost as metabolic heat when the organisms from one trophic level are consumed by organisms from the next level. There are three basic ways to transfer heat. CEnergy flows from energy sinks to energy sources. In the home as the air is heated the particles gain heat energy allowing them to move faster warming the cooler particles. The ice and the water within the first minute. Many homes are heated through the convection process which transfers heat energy through gases or liquids.
Heat moves naturally by any of three means. The sun is powered by the fusion of large atomic nuclei that creates heat and light for the solar system. Heat is the transfer of thermal energy from a system to its surroundings or from one object to another as a result of a difference in temperature. Heat is energy that flows from objects with a high. In fact all hot things radiate heat to cooler things. The processes are known as conduction convection and radiation. Heat is measured in joules J.


/heat-energy-definition-and-examples-2698981-final-2-5b76efbcc9e77c005028d736.png)



































Post a Comment for "Which Best Describes How Heat Energy Moves Within A System"